Abstract
Introduction:
Multiple myeloma (MM) is a genetically complex B cell neoplasm. Neoplastic clonal population of plasma cells acquire increasing levels of molecular aberrations including copy number variations, point mutations, gene deletions, and translocations. Guidelines define different levels of risk stratification based on genomic mutational landscape of the plasma cell in MM patients. Using genetics based information, testing aberrant pathways in individualized patients and targeting those pathways have potential for precision and personalized treatment. We summarize current literature on cancer driving mutations and signal transduction aberrant pathways.
Methods:
We searched PubMed, EMBASE, Wiley Cochrane library, Scopus, Web of Science, CINAHL, and Clinicaltrials.gov (updated July 29th, 2017) for original articles regarding MM using keywords like cytogenetics, molecular profiling, mutations, disrupted, transduction pathways, prognosis and precision medicine. We found 154 articles. After detailed scrutiny, 27 articles were included in the study.
Results:
We summarized literature search results under three categories. 1: Genetic mutations in Myeloma 2: Mutation based risk stratification 3: Aberrant intracellular transduction pathways and novel drugs targeting pathways.
Conclusion:
1: Genetic mutations in Myeloma:
Cytogenetic aberrations in MM can be divided into two main groups: translocations involving the Immunoglobulin heavy locus (IGH) at 14q32.33 and genomic imbalances, defined as hyperdiploid and nonhyperdiploid subtypes. Hyperdiploid tumors are characterized by odd number chromosomal trisomy: 3, 5, 7, 9, 11, 15, 19, and 21. Nonhyperdiploid tumors harbor IGH translocations, mainly t(4;14), t(6;14), t(11;14), t(14;16), and t(14;20). Secondary mutational events contribute to tumor progression and can include: MYC rearrangements, copy number variations (CNV): del(13q), dup(1q), del(1p) del(17p) and somatic mutations in KRAS, NRAS, BRAF, P53, and many others. Co-occurrence of two adverse genetic lesions, termed "double-hit", is specifically associated with adverse outcome.
2: Mutation based risk stratification:
MM is divided into high, intermediate and standard risk (mainly based on interphase FISH). High risk MM has Del (17p), t(14;16), t(14;20) and gene expression profiling (GEP) shows high risk signature. Intermediate risk have t(4;14), 1q gain, Cytogenetic Del 13, Hypodiploidy and PCLI ≥ 3%. Standard risk patients have t(11;14) and t(6;14). R-ISS and mSMART risk stratification guidelines rely on interphase FISH and serum biomarkers but many characterized aberrations in MM are not adequately surveyed by these methods. The use of massively parallel sequencing and array technologies to identify specific genetic mutations such as whole-exome sequencing (WES), single nucleotide polymorphism (SNP) arrays, gene expression profiling (GEP), Plasma cell labeling index (PCLI), array comparative genomic hybridization (aCGH), whole-genome sequencing (WGS)/whole exome sequecing (WES) (MALDI-TOF, NGS) in addition to historical methods can identify new subgroups and novel targetable pathways.
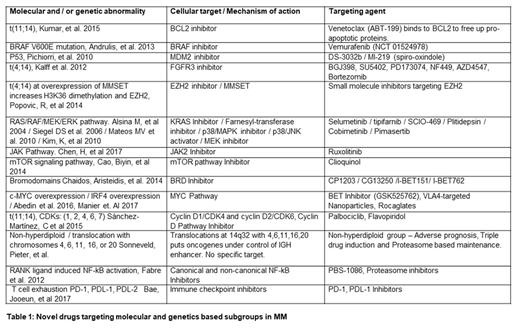
3: Aberrant transduction pathways:
Genetic aberrations lead to neoplastic proliferation and following aberrant pathways are detected in MM. KRAS/NRAS/BRAF mutations are detectable in up to 50% of newly diagnosed MM patients, making it a major therapeutic target (Walker et al 2015). Dysregulation of a Cyclin D gene, appears to be an early and unifying event in MM pathogenesis (Bergsagel et al., 2005). Pathological levels of MMSET (a histone methyltransferase) are found in t(4;14) MM cells, inhibition of MMSET would be of benefit in t(4;14) translocations. (Martinez-Garcia at al. 2011). Loss of CYLD enhances MM aggressiveness through Wnt pathway activation. WGS revealed V600E activating mutation in BRAF kinase (Andrulis et al 2013). NF-kB pathway remains constitutively active in MM and Proteasome inhibitors (Richardson et al., 2003) act principally by inhibiting NF-kB (Rajkumar et al., 2005). T cell exhaustion in MM via PD-1, PDL-1, PDL-2 signaling (Bae, Jooeun, et al 2017) can be blocked with immunecheck point inhibitors. Over active JAK Pathway (Chen, H, et al 2017) can be targeted with JAK2 Inhibitor Ruxolitinib.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal